clinical trial malaysia
Last year infectious diseases topped new sponsored clinical research in Malaysia with 40 studies reversing the trend since 2012 when oncology comprised the majority of. The Total Health Expenditure THE for Malaysia during 1997-2013 ranged from RM8303 million in 1997 to.
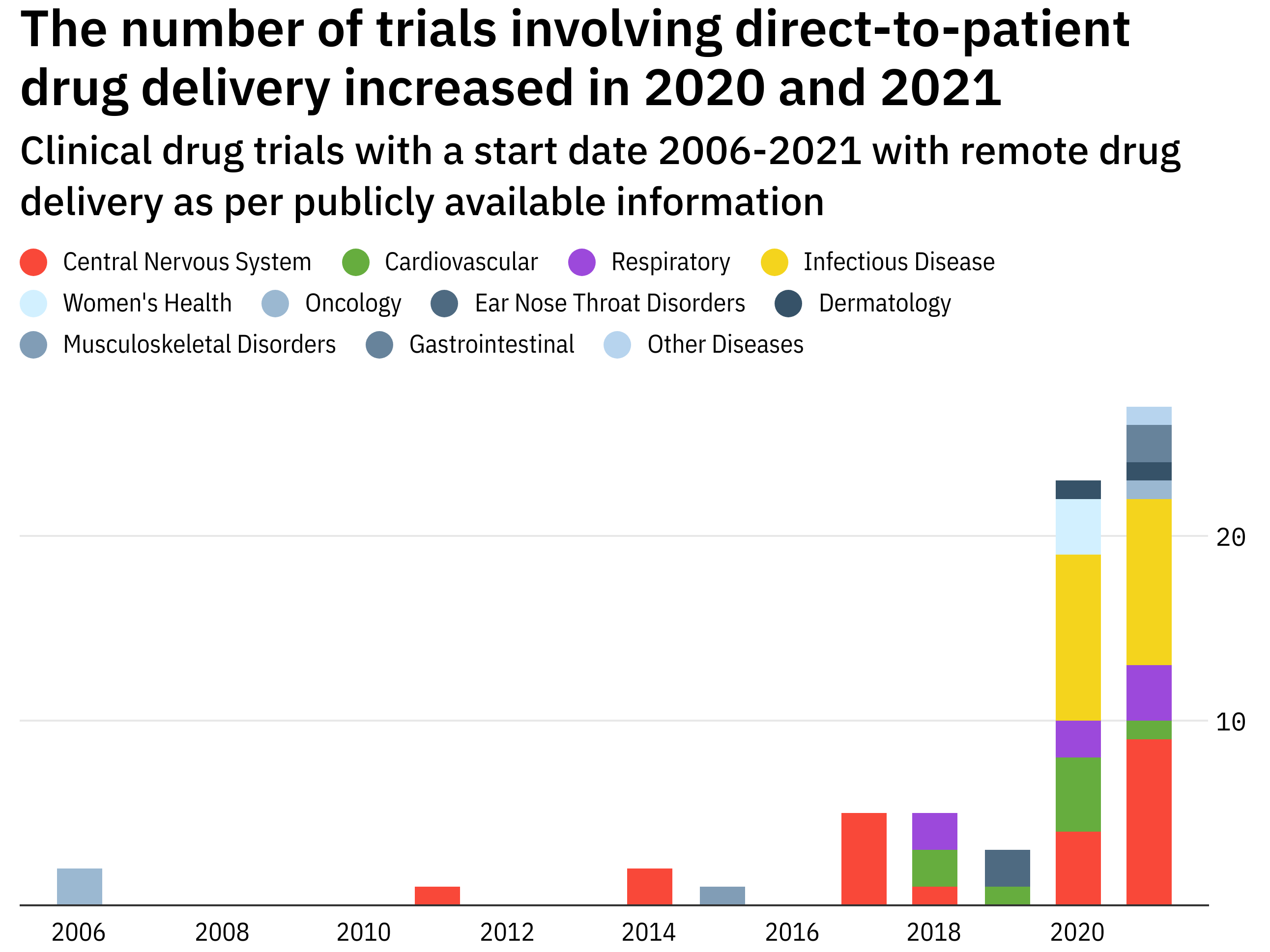
Direct To Patient The Rocky Road To Remote Drug Delivery In Clinical Trials
Besides early phase clinical research we also.
. What is the regulatory authority with oversight for clinical trial in Malaysia. DEFINITION This Guideline adopts the following. DAY 1 Virtual Session.
712A GUIDE TO CONDUCTING CLINICAL TRIALS IN MALAYSIA Fig 2. For more details please click here. Under Regulation 36B of the Pharmacy and Poisons Regulations a certificate for Clinical TrialMedicinal Test CTC is required before conducting a clinical trial.
Nach Clinical trial-Jobs in Malaysia mit Bewertungen und Gehältern suchen. Overview of GCP and Clinical Research in Malaysia. International Council of Harmonization ICH.
Malaysian Guideline for Application of Clinical Trial Import Licence and Clinical Trial Exemption National Pharmaceutical Control Bureau Ministry of Health Malaysia Malaysian Guideline for. Malaysia has a single regulatory authority the National Pharmaceutical Control Bureau NPCB. Research and Clinical Trials Malaysian Oncological Society Healthcare Professionals Research and Clinical Trials The following are Industry Sponsored Clinical.
A properly planned and executed clinical trial is a powerful experimental technique for assessing the effectiveness of an intervention. The single regulatory authority in malaysia that regulates clinical trials and the licensing scheme for clinical trials is the npra1the national committee for clinical research. 45 Jobs für Clinical trial in Malaysia.
The current composition of the NCCR was established to ensure that it becomes visionary and pro-active in driving the development of clinical research in the. Centre for Clinical Trial CCT was established in 2010 to conduct and support early and late phase clinical research in Malaysia. General Clinical Trial.
Key features of the Malaysian clinical trial landscape include streamlined submission and regulatory processes in English quick start-up times a strong network of experienced KOLs. Malaysia has inherent benefits to conduct clinical trials such as its large multi-ethnic population that offers genetic diversity Established and good public and private healthcare systems A. Malaysias multi-ethnic population disease burden medical infrastructure and research speed combined with its populations proficiency in English make the Southeast.
National Committee for Clinical Research A. On August 2020 the NPRA of Malaysia has updated a document intended to guide the applicant in making Clinical Trial Import Licence CTIL and Clinical Trial Exemption CTX applications to. A clinical trial conducted according to a single protocol but at more than one site and therefore carried out by more than one investigator.
Listing a study does not mean it has. This clinical trial was designed to determine the efficacy of vitamin D supplementation on plasma 25-hydroxyvitamin D 25OHD and intact parathyroid hormone PTH concentrations of. Malaysian investigators have over the past decade been involved in major clinical outcome trials which were subsequently published in major medical journals.
The Centre for Investigational New Product is the unit in charge. Hong Kong Indonesia Malaysia the Philippines Singapore Taiwan Thailand and Vietnam. HCV Self-testing in Malaysia The safety and scientific validity of this study is the responsibility of the study sponsor and investigators.
A The 2007 directive requiring all ethics committees that approve clinical trials in Malaysia to be registered with the Malaysian Drug Control Authority DCA a body established under the. In most cases the smaller Asian countries will not require local clinical studies and.
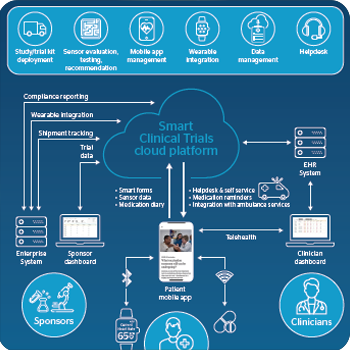
Accelerating Clinical Trials And Reducing Costs With Digital Technologies Atos
National Covid 19 Immunisation Programme News From Mission Portal
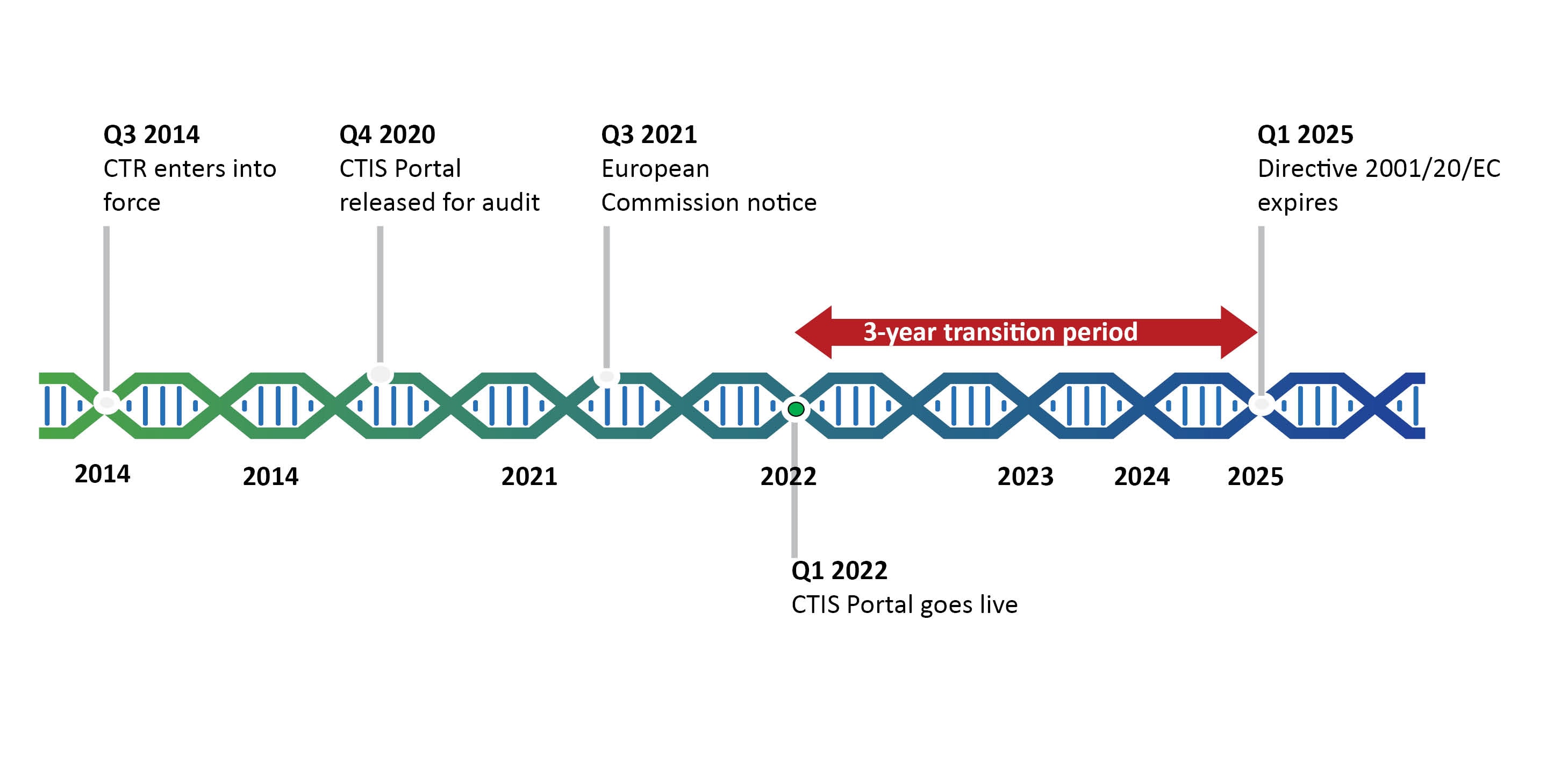
Introduction To The Clinical Trials Regulation Deloitte Netherlands

Institute For Clinical Research Icr Nih My Icr Nih Twitter

Ian Rentsch Vice President Head Global Data Partnerships Exec Leadership Team Member Trinetx Linkedin

Hospital Serdang Clinical Research Malaysia

Clinical Research Services For Early Stage Biotech Companies

Institute Of Clinical Research Linkedin
Clinical Trial Logistics Improving Compliance Efficiency Parexel

Clinical Research Services For Early Stage Biotech Companies

National Medical Research Register

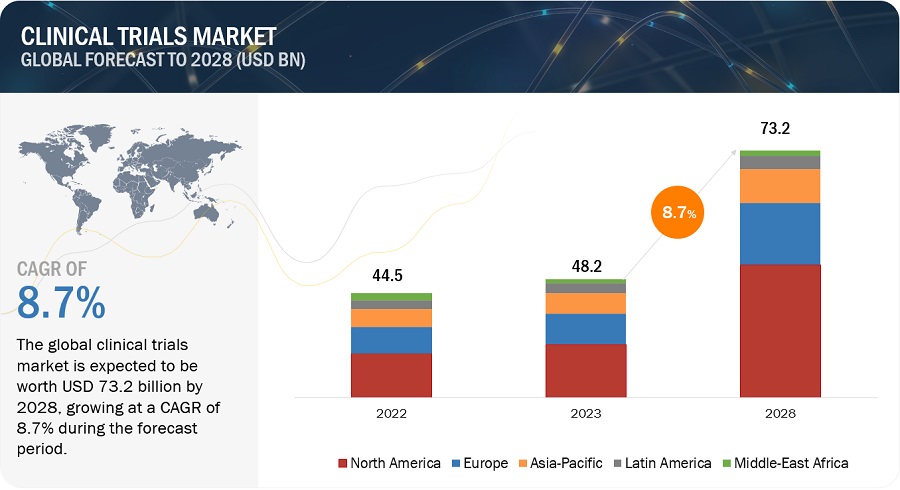
Clinical Trials Market Size Share 2022 2026 Marketsandmarkets

Innovation In Clinical Trial Methodologies 1st Edition
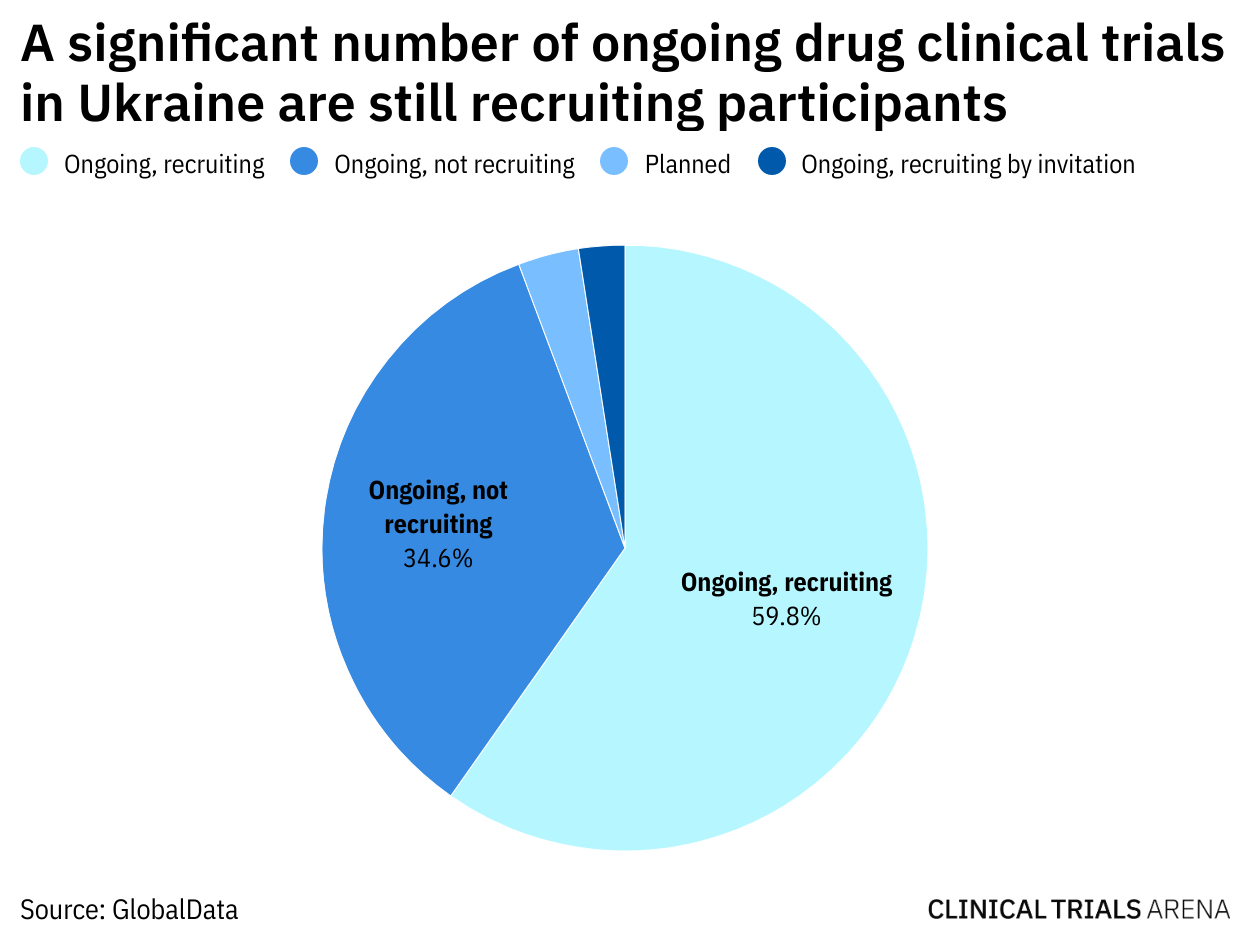
Ukraine Industry Sponsored Clinical Trial Development At Risk

Hospital Ampang Clinical Research Malaysia
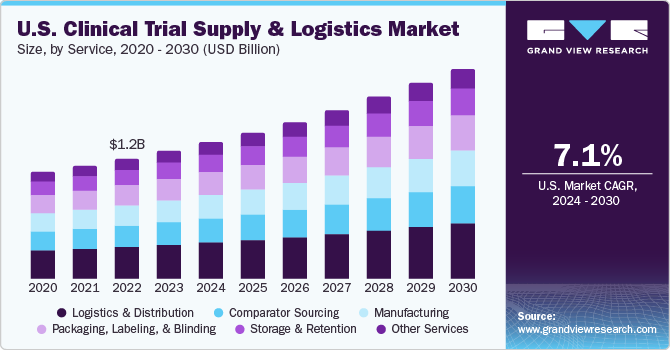
Clinical Trial Supply And Logistics Market Report 2028

Introduction To The Clinical Trials Regulation Deloitte Netherlands


Comments
Post a Comment